-
PDF
- Split View
-
Views
-
Cite
Cite
Walter Giordano, Carlos E. Domenech, Aeration affects acetate destination in Gibberella fujikuroi, FEMS Microbiology Letters, Volume 180, Issue 1, November 1999, Pages 111–116, https://doi.org/10.1111/j.1574-6968.1999.tb08784.x
Close - Share Icon Share
Abstract
Gibberellins, fatty acids and the polyketides bikaverin and fusarin C are synthesized from a common precursor, acetyl-CoA. The production of these compounds in Gibberella fujikuroi was strongly influenced by aeration, determined by the air/medium ratio in shaken batch cultures. Higher aeration resulted in increased growth and the production of bikaverin and gibberellins. Low aeration stimulated fatty acid and fusarin C production. Feeding experiments with labeled leucine or acetate resulted in different proportions of labeled palmitic acid and bikaverin, indicating that these compounds are synthesized from independent acetate pools.
1 Introduction
The fungus Gibberella fujikuroi (perfect stage of Fusarium moniliforme) produces gibberellins [1] and other secondary metabolites such as carotenoids [2], bikaverin [3], and fusarin C [4].
Gibberellins and carotenoids are terpenoid compounds that share biosynthetic pathways, but their biosynthesis is physically separated into different subcellular compartments [5]. The most important end products of gibberellin and carotenoid biosynthesis in G. fujikuroi are gibberellic acid [6] and neurosporaxanthin [2], respectively. Bikaverin and fusarin C are produced from acetyl-CoA via the polyketide pathway. Although the synthesis of bikaverin and gibberellins responds to various combinations of signals such as nitrogen availability, nitrate and the pH of the medium, neither metabolic pathway is subject to common regulation [7].
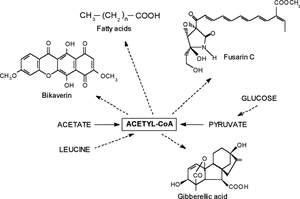
G. fujikuroi also accumulates fatty acids and excretes fatty acid esters to the culture medium [4]. Long-chain fatty acids are synthesized from acetyl-CoA through a biochemical pathway whose initial steps are quite similar to those of polyketide biosynthesis [8]. Thus, the biosyntheses of bikaverin, fusarin C, fatty acids and gibberellins have acetyl-CoA as a common precursor, through the polyketide, fatty acid or the terpenoid pathway (Fig. 1). Our results show that aeration of the culture plays a major role in channeling acetyl-CoA into the biosynthesis of these different compounds.
Biosynthesis of gibberellins, fatty acids, fusarin C and bikaverin from acetyl-CoA.
2 Materials and methods
2.1 Strains and culture conditions
The wild-type strain IMI58289 of G. fujikuroi, obtained from the Commonwealth Mycological Institute (Kew, UK), was used throughout this work. The cultures were grown from spores, as described previously [7]. The effect of aeration was studied by varying the proportion between air and culture medium in the flasks [9]; the cultures were incubated in 125-ml Erlenmeyer flasks containing 25, 50, 75 or 100 ml of medium. The flasks with 25 and 100 ml of medium are referred to hereafter as high- and low-aeration media, respectively. Dry weights and glucose concentrations were determined as described in [10].
2.2 Radioactive labeling
Sodium [2-14C]acetate (2.0×1012 Bq nmol−1, New England Nuclear) was combined with non-radioactive sodium acetate, and uniformly labeled l-[14C]leucine (1.1×1013 Bq nmol−1, New England Nuclear) was combined with non-radioactive l-[14C]leucine to give a final concentration of 10 mM and 4.4×108 Bq nmol−1. The two compounds were sterilized by filtration and added to autoclaved minimal medium.
The radioactivity in bikaverin and palmitic acid was measured by scintillation counting after collection of the corresponding fractions as described below. The specific radioactivity in these compounds was estimated according to [5]. The maximum expected specific radioactivities for bikaverin and palmitic acid were calculated on the assumption that they were derived exclusively from the radioactive precursor with a specific radioactivity of 0.44 Bq nmol−1. Assuming that uniformly labeled leucine produced radioactive acetate, as acetyl-CoA labeled in carbons 1 and 2, and that bikaverin and palmitic acid were derived exclusively from labeled acetate, the maximal specific radioactivity expected was n times the specific radioactivity of each carbon of the precursor (Table 1) where n is the number of carbon atoms in the product derived from the labeled carbon of the precursor.
Specific radioactivity of palmitic acid and bikaverin after 7 days growth of G. fujikuroi in minimal medium supplemented with 10 mM l-[U-14C]leucine or sodium [2-14C]acetate
| Compound | l-[U-14C]Leucine | [2-14C]Acetate | ||||||
| n | Radioactivity | n | Radioactivity | |||||
| Bq nmol−1 | % | Bq nmol−1 | % | |||||
| maximal | found | maximal | found | |||||
| Palmitic acid | 16 | 1.17 | 0.11±0.01 | 9 | 8 | 3.52 | 0.27±0.01 | 8 |
| Bikaverin | 18 | 1.32 | 0.30±0.02 | 23 | 9 | 3.96 | 0.09±0.01 | 2 |
| Compound | l-[U-14C]Leucine | [2-14C]Acetate | ||||||
| n | Radioactivity | n | Radioactivity | |||||
| Bq nmol−1 | % | Bq nmol−1 | % | |||||
| maximal | found | maximal | found | |||||
| Palmitic acid | 16 | 1.17 | 0.11±0.01 | 9 | 8 | 3.52 | 0.27±0.01 | 8 |
| Bikaverin | 18 | 1.32 | 0.30±0.02 | 23 | 9 | 3.96 | 0.09±0.01 | 2 |
The percent refers to the maximal expected specific radioactivity.
Specific radioactivity of palmitic acid and bikaverin after 7 days growth of G. fujikuroi in minimal medium supplemented with 10 mM l-[U-14C]leucine or sodium [2-14C]acetate
| Compound | l-[U-14C]Leucine | [2-14C]Acetate | ||||||
| n | Radioactivity | n | Radioactivity | |||||
| Bq nmol−1 | % | Bq nmol−1 | % | |||||
| maximal | found | maximal | found | |||||
| Palmitic acid | 16 | 1.17 | 0.11±0.01 | 9 | 8 | 3.52 | 0.27±0.01 | 8 |
| Bikaverin | 18 | 1.32 | 0.30±0.02 | 23 | 9 | 3.96 | 0.09±0.01 | 2 |
| Compound | l-[U-14C]Leucine | [2-14C]Acetate | ||||||
| n | Radioactivity | n | Radioactivity | |||||
| Bq nmol−1 | % | Bq nmol−1 | % | |||||
| maximal | found | maximal | found | |||||
| Palmitic acid | 16 | 1.17 | 0.11±0.01 | 9 | 8 | 3.52 | 0.27±0.01 | 8 |
| Bikaverin | 18 | 1.32 | 0.30±0.02 | 23 | 9 | 3.96 | 0.09±0.01 | 2 |
The percent refers to the maximal expected specific radioactivity.
2.3 Quantitative determination of bikaverin, fusarin C, fatty acids and gibberellins
Bikaverin concentration was determined spectroscopically. To determine its specific radioactivity the protocol utilized previously was followed [7]. Fusarin C was extracted from the filtered medium by partition with chloroform, and its concentration determined by UV spectroscopy at 350 nm; ε of fusarin C in methanol=25 000 [4]. Overall gibberellin concentrations in the medium were estimated with a fluorometric method [10].
Fatty acids were extracted with hexane from culture medium after acidification to pH 2.5 with HCl. Mycelia were collected by filtration, washed with water, ground in a mortar, extracted with hexane and cleared by centrifugation. The fatty acid extracts were concentrated and methylated by incubation at 60°C for 2 h with 9:1 methanol:benzene and 2% H2SO4. The fatty acid methyl esters were extracted with hexane and analyzed with a Hewlett Packard gas chromatograph (model 5890, series II, equipped with a Hewlett-Packard capillary column HP-1 of cross-linked methylsilicone gum, 25 m×0.2 mm×0.33 µm film thickness) programmed for 10 min at 185°C increasing to 220°C at 4°C min−1; injector temperature 250°C; flame ionization detector at 300°C; carrier gas N2 at 1 ml min−1. To determine the radioactivity in palmitic acid, HPLC analyses were carried out in a Waters chromatograph, model 600E [11].
3 Results
3.1 Growth kinetics in different volumes of medium
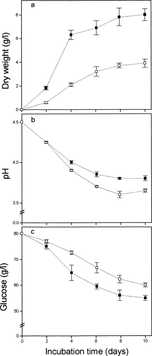
Growth and metabolism of G. fujikuroi differed according to the aeration of the culture (Fig. 2). Under low aeration there was less biomass production than in a high air/medium ratio, and growth remained linear longer under low aeration (6 days) than under high aeration (4 days) (Fig. 2a). There was a drop in the external pH with aging of the culture under both aeration conditions (Fig. 2b). The pH decrease was somewhat more pronounced under low-aeration conditions. Glucose consumption was enhanced under high-aeration conditions (Fig. 2c), but in terms of mycelium dry mass, consumption of glucose was favored by the lower degree of aeration. For example, considering the values shown in Fig. 2a,c, in a 10-day culture, the glucose consumption was approximately 5.3 or 3.2 g per g of dry mycelium, for low and high aeration, respectively.
Mycelial dry weight (a), changes in pH (b), and glucose concentrations (c) in cultures with different aeration. ▪, 100 ml of air; □, 25 ml of air.
3.2 Terpenoids, polyketides and fatty acid production
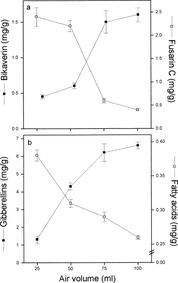
The production of bikaverin was increased in high-aerated cultures, while fusarin C was the predominant polyketide under low aeration (Fig. 3a). In a similar manner, gibberellin production was enhanced by high aeration, while fatty acid accumulation was reduced (Fig. 3b).
Gibberellin, fatty acid, fusarin C and bikaverin accumulation in cultures with different aeration. The cultures contained 25, 50, 75 and 100 ml of liquid medium and were grown in 125-ml Erlenmeyer flasks for 7 days.
Feeding experiments with radioactively labeled l-leucine resulted in the production of labeled palmitic acid and bikaverin, indicating that l-leucine is a source of acetate in the cell. Addition of labeled leucine or acetate to the culture resulted in the accumulation of different proportions of labeled palmitic acid and bikaverin (Table 1). l-Leucine proved to be a better source for the synthesis of bikaverin than for palmitic acid. Acetate provided about 8% of the carbon atoms in palmitic acid, and a very small proportion of those in bikaverin.
4 Discussion
The level of aeration affects the metabolism of G. fujikuroi grown in batch cultures. High aeration stimulates growth and leads to a lower acidification of the medium and glucose consumption, indicating an increase in aerobic metabolism to the detriment of the anaerobic process. The aeration level affects the acetate-derived secondary metabolism of G. fujikuroi in different ways. Low aeration should impair acetate consumption via the citric acid cycle. As a consequence, more acetate should be available for the synthesis of acetate derivatives such as terpenoids and polyketides. This was true for fatty acid and fusarin C biosynthesis. In a similar way, aflatoxin and poly-β-hydroxybutyrate production was stimulated by low oxygen tension in Aspergillus parasiticus[9] and Azotobacter beijerinckii[12], respectively. The opposite effect was found in the case of bikaverin and gibberellin biosynthesis, which was reduced under low-aeration conditions. A similar situation was found in carotenoid and polyhydroxybutyrate biosynthesis in Azospirillum brasilense[13], whose production from acetate decreased and increased, respectively, under low-aeration conditions. In terms of polyketide biosynthesis, the decrease in bikaverin production in low-aerated cultures of G. fujikuroi was compensated by the increase in fusarin C, suggesting a specific aeration-sensitive regulation that controls which polyketide is produced.
The enhanced fatty acid accumulation under low-aeration conditions suggests that, as shown in Aspergillus nidulans[14], G. fujikuroi may contain two functionally distinct fatty acid synthases, one required for primary fatty acid metabolism and another for secondary metabolism. Both enzymes could be regulated by oxygen in an opposite fashion, as occurs in the case of the two hydroxymethylglutaryl-CoA reductase enzymes in Saccharomyces cerevisiae[15].
Feeding experiments with labeled substrates revealed differences between fatty acid and bikaverin biosynthesis. Acetate was more effective than leucine in the production of palmitic acid. Leucine, however, a compound capable of producing acetyl-CoA and acetoacetic acid, proved to be a better source for bikaverin biosynthesis than for fatty acids. The ability of G. fujikuroi to utilize these exogenous carbon sources to synthesize bikaverin and fatty acids indicates that the biosynthesis of these compounds employs different acetate pools as precursors. Similar results showed that three different terpenoids, gibberellins, carotenoids and sterols, are synthesized in different cellular compartments [5]. This appears to be the case in plant cells also, where different pools of acetyl-CoA are generated in plastids, chloroplasts and mitochondria [16].
As a whole, our results indicate that oxygen, per se or through a metabolite, affects acetate utilization in G. fujikuroi. This regulation may imply specific regulatory effects on key enzymes of the different metabolic pathways or, alternatively, it may control the access of acetate to the different compartments where these biosynthetic processes take place.
Acknowledgements
This work was supported by grants from CONICOR and CONICET of the República Argentina. C.E.D. is a Career Member of CONICET and W.G. is a fellow of CONICET. We gratefully acknowledge Dr. Javier Avalos and Dr. Enrique Cerdá-Olmedo for critical reading of the manuscript and Mrs. D. Balegno for help in its English preparation.
References