-
PDF
- Split View
-
Views
-
Cite
Cite
Jacqueline A Sayer, Martin Kierans, Geoffrey M Gadd, Solubilisation of some naturally occurring metal-bearing minerals, limescale and lead phosphate by Aspergillus niger, FEMS Microbiology Letters, Volume 154, Issue 1, September 1997, Pages 29–35, https://doi.org/10.1111/j.1574-6968.1997.tb12620.x
Close - Share Icon Share
Abstract
The ability of the soil fungus Aspergillus niger to tolerate and solubilise seven naturally occurring metal-bearing minerals, limescale and lead phosphate was investigated. A. niger was able to solubilise four of the test insoluble compounds when incorporated into solid medium: cuprite (CuO2), galena (PbS), rhodochrosite (Mn(CO3)x) and limescale (CaCO3). A. niger was able to grow on all concentrations of all the test compounds, whether solubilisation occurred or not, with no reduction in growth rate from the control. In some cases, stimulation of growth occurred, most marked with the phosphate-containing mineral, apatite. Precipitation of insoluble copper and manganese oxalate crystals under colonies growing on agar amended with cuprite and rhodochrosite was observed after 1–2 days growth at 25°C. This process of oxalate formation represents a reduction in bioavailability of toxic cations, and could represent an important means of toxic metal immobilisation of physiological and environmental significance.
1 Introduction
The solubilisation of insoluble metal compounds by fungi has biotechnological applications, e.g. the reclamation of metals from low-grade ores and the recovery of metals from industrial by-products [1]. Solubilisation usually occurs as a result of protonation of the anion of the metal compound by mechanisms that include proton efflux and organic acid production [2]. The proton translocating ATPase of the plasma membrane generates the electrochemical gradients that are required for the acquisition of nutrients by active efflux of protons into the external medium [3–5]. The production of organic acids provides both a source of protons and an organic acid anion, the latter often capable of forming a complex with the metal cation which affects mobility and toxicity [6, 7]: citric acid increases the environmental mobility of many potentially toxic metals [8]. Fungi have been isolated from weathered sandstone where 28% of the isolates produced organic acids: Penicillium corylophillum was able to produce oxalic acid when the only source of carbon and nitrogen was derived from the alga Monoraphidium braunii[9]. Oxalate is the most studied ligand as it can form strong complexes with aluminium and enhance silicate dissolution [10]. The weathering of rocks and minerals is accelerated in the presence of plants and microorganisms, especially in the microenvironments of roots and hyphae [11], and solubilisation activity by means of organic acid production is a major factor in this process. Solubilisation can also occur by the production of siderophores, which are Fe(III)-specific bidentate ligands used by microorganisms to accumulate iron, also having a major incidental role in Fe(III) weathering. Iron has been solubilised from goethite, biotite and pyrite by the production of siderophores [12] and purified bacterial siderophores have been reported to be able to leach 10−8 mol Fe m−2 h−1 hematite [13].
In this work, the solubilisation of seven naturally occurring metal-bearing minerals, limescale (CaCO3) and lead phosphate by Aspergillus niger was examined. A. niger was tested for the ability to tolerate and/or solubilise commercially available lead phosphate, and galena (PbS, included in the seven mineral compounds screened) because it is known that although the mineralogical form of lead in soil can affect toxicity [14], lead inhibits soil microbial respiration, and therefore the decomposition of soil organic matter [15]. A method of screening fungal strains for their ability to solubilise insoluble metal compounds was used, based on observing clear zones around fungal colonies growing on agar medium amended with selected insoluble metal compounds [16]. This method also provides information on relative toxicities and the metal tolerance of fungal strains by comparison of growth rates. We have demonstrated that A. niger is capable of the solubilisation of selected metal-bearing minerals and compounds and, in some cases, can transform the substance into an insoluble metal oxalate via an intermediate solubilisation stage.
2 Materials and methods
2.1 Preparation of metal-containing minerals and X-ray diffraction analysis
Seven metal-containing mineral ores [apatite (Ca5(PO4)3), chalcopyrite (CuFeS2), cuprite (CuO2), galena (PbS), rhodochrosite (Mn(CO3)x), selenite [CaSO4·2H2O] and sphalerite (ZnS)] were obtained from R.G. Widdowson (Scarborough, UK). These minerals were powdered in a ball mill, and composition and purity verified by powder X-ray diffraction using a Hilton-Brooks X-ray diffractometer fitted with a curved graphite monochromator, using Cu Kα 1° radiation. A sample of limescale (CaCO3, from Garway Hill, Herefordshire, UK) was dried to constant weight at 60°C and ground in a pestle and mortar. Pb3(PO4)2 was obtained from Alfa (Johnson Matthey, Royston, UK).
2.2 Organism, media and culture conditions
The organism used was Aspergillus niger (from our own culture collection), maintained on malt extract agar (MEA, lab M) at 25°C. All experiments were carried out using MEA with the addition of the appropriate metal compound to the desired final concentration. It should be noted that the concentrations of the insoluble metal compounds are expressed here in molar terms, which does not provide any information on the partitioning of the metal between soluble and insoluble forms: it should be noted that a natural equilibrium will be reached between these phases, and that soluble cations may bind to medium components.
A. niger was inoculated, in triplicate, onto 10 cm3 MEA containing the powdered metal-containing minerals, limescale or lead phosphate, in 90 mm diameter Petri dishes. The metal-bearing minerals were used over a concentration range of 0–100 mM. Powdered limescale was incorporated into the agar at concentrations of 0–0.5% (w/v). Pb3(PO4)2 was incorporated into the agar at concentrations of 2.5, 5 and 10 mM. Inoculations were carried out with 7 mm diameter discs of mycelium cut from colonies which had been grown on MEA at 25°C for at least 24 h. Inoculated plates were incubated at 25°C, and daily measurements were made of the diameter of the colonies any zones of solubilisation present. Rates of colony extension and extension of the solubilised zone were calculated by least squares regression over the linear portion of the data.
2.3 Crystal formation in agar under colonies
When A. niger was grown on agar amended with cuprite and rhodochrosite, crystals formed in the agar under and around the colonies after 1–2 days growth at 25°C. These crystals were extracted by gently homogenising the agar in approximately 50 ml warm distilled deionised water (ddH2O) in a crystallising dish. The crystals were allowed to settle to the bottom of the dish, and the aqueous phase was removed [6]. Small samples of the crystals were mounted on double-sided carbon adhesive tape on 7 mm diameter aluminium stubs and dried overnight in a vacuum desiccator. The crystals were examined using a JEOL JSM-35 scanning electron microscope (SEM) coupled with energy dispersive X-ray microanalysis (EDXA, link interface p1449). For SEM, the samples were sputter-coated for 5 min using a Polaron E5100 series II ‘cool’ sputter coater fitted with a Au/Pd target. For EDXA, uncoated samples were analysed for at least 100 s at a voltage of 15 kV.
3 Results
3.1 Solubilisation of naturally occurring metal-bearing minerals, limescale and lead phosphate by A. niger
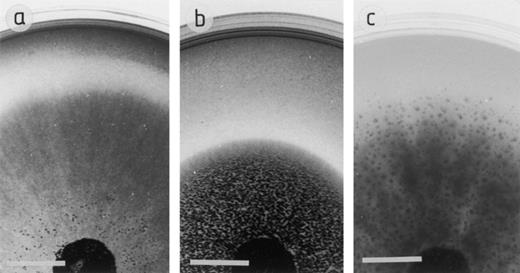
The results of the powder X-ray diffraction showed that apatite [Ca5(PO4)3(OH)], chalcopyrite (CuFeS2), cuprite (CuO2), galena (PbS), rhodochrosite (Mn(CO3)x), selenite [Ca(SeO3)] and sphalerite (ZnS) were all pure samples of the mineral (results not shown). Solubilisation of galena and cuprite by A. niger is shown in Fig. 1. The results in Table 1Table 2 are presented as ratios of the colony extension rate in the presence of a given metal compound (Rm) to that of the control (Rc), and the rate of extension of the clear zone of solubilisation (Rs) in relation to the extension rate of that colony (Rm). A ratio of 1.0 indicates that the colony extension rate in the presence of a metal compound (Rm) is the same as the control extension rate (Rc) and that the rate of extension of the clear zone of solubilisation (Rs) on a given metal compound is the same as the colony extension rate (Rm). A. niger was able to grow on all the minerals tested, with no apparent reduction in growth rate. There was a slight stimulation of growth by galena, cuprite and chalcopyrite, and an approximate 50% stimulation of growth with apatite on all concentrations tested. As the concentration of apatite increased, growth stimulation increased, probably due to the presence of released phosphate. A. niger partially solubilised cuprite and galena (the zone of solubilisation was not completely clear) (see Fig. 1). There was no zone of solubilisation around colonies growing on rhodochrosite, but a zone of crystal formation was clearly visible under the colony (Fig. 1). Selenite was only solubilised at a concentration of 40 mM, although the selenite was extremely difficult to see in the agar at the lower concentrations: any solubilisation at a concentration of 20 mM may not have been discerned. The solubilisation of limescale and lead phosphate is shown in Table 2. Limescale (at 0.3–0.5% w/v) greatly enhanced the growth of A. niger to more than double that of the control. The limescale was solubilised at all concentrations tested, the fastest rate of solubilisation being at the lower concentrations. Lead phosphate did not affect the growth rate of A. niger, and was either only partially solubilised, or immediately re-precipitated [17].
Growth of A. niger on (a) 60 mM galena, (b) 60 mM cuprite and (c) 60 mM rhodochrosite when incorporated into MEA. In (a) and (b), the clear zone of solubilisation can be seen at the colony edge; in (c), there is no zone of solubilisation, but extensive crystal formation under the colony. The photographs were taken after 6 days growth at 25°C, scale bar=1 cm.
Growth on and solubilisation of metal-bearing minerals (apatite, chalcopyrite, cuprite, galena, rhodochrosite, selenite and sphalerite) in MEA by A. niger
| 20 mM | 40 mM | 60 mM | 80 mM | 100 mM | |
| Apatite | |||||
| Rm:Rc | 1.54 | 1.61 | 1.62 | 1.62 | 1.65 |
| Rs:Rm | – | – | – | – | – |
| Chalcopyrite | |||||
| Rm:Rc | 1.21 | 1.15 | 1.15 | 1.16 | 1.20 |
| Rs:Rm | – | – | – | – | – |
| Cuprite | |||||
| Rm:Rc | 1.31 | 1.27 | 1.10 | 1.02 | 0.91 |
| Rs:Rm | 1.33b | 0.63b | 1.03b | 1.03b | 1.04b |
| Galena | |||||
| Rm:Rc | 1.20 | 1.24 | 1.21 | 1.20 | 1.23 |
| Rs:Rm | 1.21a | 1.15a | 0.91a | 1.08a | 1.04a |
| Rhodochrosite | |||||
| Rm:Rc | 1.06 | 1.12 | 1.10 | 1.10 | 1.15 |
| Rs:Rm | – | 1.06c | 1.09c | 1.07c | 1.05c |
| Selenite | |||||
| Rm:Rc | 1.07 | 1.08 | 1.10 | 1.10 | 1.11 |
| Rs:Rm | – | 0.93 | – | – | – |
| Sphalerite | |||||
| Rm:Rc | 1.05 | 1.05 | 1.04 | 1.05 | 1.08 |
| Rs:Rm | – | – | – | – | – |
| 20 mM | 40 mM | 60 mM | 80 mM | 100 mM | |
| Apatite | |||||
| Rm:Rc | 1.54 | 1.61 | 1.62 | 1.62 | 1.65 |
| Rs:Rm | – | – | – | – | – |
| Chalcopyrite | |||||
| Rm:Rc | 1.21 | 1.15 | 1.15 | 1.16 | 1.20 |
| Rs:Rm | – | – | – | – | – |
| Cuprite | |||||
| Rm:Rc | 1.31 | 1.27 | 1.10 | 1.02 | 0.91 |
| Rs:Rm | 1.33b | 0.63b | 1.03b | 1.03b | 1.04b |
| Galena | |||||
| Rm:Rc | 1.20 | 1.24 | 1.21 | 1.20 | 1.23 |
| Rs:Rm | 1.21a | 1.15a | 0.91a | 1.08a | 1.04a |
| Rhodochrosite | |||||
| Rm:Rc | 1.06 | 1.12 | 1.10 | 1.10 | 1.15 |
| Rs:Rm | – | 1.06c | 1.09c | 1.07c | 1.05c |
| Selenite | |||||
| Rm:Rc | 1.07 | 1.08 | 1.10 | 1.10 | 1.11 |
| Rs:Rm | – | 0.93 | – | – | – |
| Sphalerite | |||||
| Rm:Rc | 1.05 | 1.05 | 1.04 | 1.05 | 1.08 |
| Rs:Rm | – | – | – | – | – |
Results are presented in the form of ratios of the rate of growth on mineral-amended media in relation to growth on control media (Rm:Rc) and the rate of extension of the solubilised zone in relation to growth on that given mineral (Rs:Rm). Control growth rate for A. niger= 7.59 (±0.11) mm day−1 (average of 3 replicates). –, no solubilisation. aPartial solubilisation only. bCrystallisation under colony, and in zone of solubilisation. cCrystallisation under colony, but no zone of solubilisation.
Growth on and solubilisation of metal-bearing minerals (apatite, chalcopyrite, cuprite, galena, rhodochrosite, selenite and sphalerite) in MEA by A. niger
| 20 mM | 40 mM | 60 mM | 80 mM | 100 mM | |
| Apatite | |||||
| Rm:Rc | 1.54 | 1.61 | 1.62 | 1.62 | 1.65 |
| Rs:Rm | – | – | – | – | – |
| Chalcopyrite | |||||
| Rm:Rc | 1.21 | 1.15 | 1.15 | 1.16 | 1.20 |
| Rs:Rm | – | – | – | – | – |
| Cuprite | |||||
| Rm:Rc | 1.31 | 1.27 | 1.10 | 1.02 | 0.91 |
| Rs:Rm | 1.33b | 0.63b | 1.03b | 1.03b | 1.04b |
| Galena | |||||
| Rm:Rc | 1.20 | 1.24 | 1.21 | 1.20 | 1.23 |
| Rs:Rm | 1.21a | 1.15a | 0.91a | 1.08a | 1.04a |
| Rhodochrosite | |||||
| Rm:Rc | 1.06 | 1.12 | 1.10 | 1.10 | 1.15 |
| Rs:Rm | – | 1.06c | 1.09c | 1.07c | 1.05c |
| Selenite | |||||
| Rm:Rc | 1.07 | 1.08 | 1.10 | 1.10 | 1.11 |
| Rs:Rm | – | 0.93 | – | – | – |
| Sphalerite | |||||
| Rm:Rc | 1.05 | 1.05 | 1.04 | 1.05 | 1.08 |
| Rs:Rm | – | – | – | – | – |
| 20 mM | 40 mM | 60 mM | 80 mM | 100 mM | |
| Apatite | |||||
| Rm:Rc | 1.54 | 1.61 | 1.62 | 1.62 | 1.65 |
| Rs:Rm | – | – | – | – | – |
| Chalcopyrite | |||||
| Rm:Rc | 1.21 | 1.15 | 1.15 | 1.16 | 1.20 |
| Rs:Rm | – | – | – | – | – |
| Cuprite | |||||
| Rm:Rc | 1.31 | 1.27 | 1.10 | 1.02 | 0.91 |
| Rs:Rm | 1.33b | 0.63b | 1.03b | 1.03b | 1.04b |
| Galena | |||||
| Rm:Rc | 1.20 | 1.24 | 1.21 | 1.20 | 1.23 |
| Rs:Rm | 1.21a | 1.15a | 0.91a | 1.08a | 1.04a |
| Rhodochrosite | |||||
| Rm:Rc | 1.06 | 1.12 | 1.10 | 1.10 | 1.15 |
| Rs:Rm | – | 1.06c | 1.09c | 1.07c | 1.05c |
| Selenite | |||||
| Rm:Rc | 1.07 | 1.08 | 1.10 | 1.10 | 1.11 |
| Rs:Rm | – | 0.93 | – | – | – |
| Sphalerite | |||||
| Rm:Rc | 1.05 | 1.05 | 1.04 | 1.05 | 1.08 |
| Rs:Rm | – | – | – | – | – |
Results are presented in the form of ratios of the rate of growth on mineral-amended media in relation to growth on control media (Rm:Rc) and the rate of extension of the solubilised zone in relation to growth on that given mineral (Rs:Rm). Control growth rate for A. niger= 7.59 (±0.11) mm day−1 (average of 3 replicates). –, no solubilisation. aPartial solubilisation only. bCrystallisation under colony, and in zone of solubilisation. cCrystallisation under colony, but no zone of solubilisation.
Growth on and solubilisation of limescale and Pb3(PO4)2 by A. niger when incorporated in MEA
| Rm:Rc | Rs:Rm | |
| Limescale | ||
| 0.2% (w/v) | 1.19 | 1.67 |
| 0.3% (w/v) | 2.36 | 1.02 |
| 0.4% (w/v) | 2.38 | 1.03 |
| 0.5% (w/v) | 2.39 | 1.06 |
| Pb3(PO4)2 | ||
| 2.5 mM | 1.01 | – |
| 5.0 mM | 1.00 | 1.01a |
| 10.0 mM | 1.00 | 1.01a |
| Rm:Rc | Rs:Rm | |
| Limescale | ||
| 0.2% (w/v) | 1.19 | 1.67 |
| 0.3% (w/v) | 2.36 | 1.02 |
| 0.4% (w/v) | 2.38 | 1.03 |
| 0.5% (w/v) | 2.39 | 1.06 |
| Pb3(PO4)2 | ||
| 2.5 mM | 1.01 | – |
| 5.0 mM | 1.00 | 1.01a |
| 10.0 mM | 1.00 | 1.01a |
Results are presented in the form of ratios of the rate of growth on amended media in relation to control growth (Rm:Rc) and the rate of extension of the solubilised zone in relation to rate of growth on amended media (Rs:Rm). Control growth rate of A. niger: 7.00 (±0.49) mm day−1 (average of three replicates). aPartial solubilisation only.
Growth on and solubilisation of limescale and Pb3(PO4)2 by A. niger when incorporated in MEA
| Rm:Rc | Rs:Rm | |
| Limescale | ||
| 0.2% (w/v) | 1.19 | 1.67 |
| 0.3% (w/v) | 2.36 | 1.02 |
| 0.4% (w/v) | 2.38 | 1.03 |
| 0.5% (w/v) | 2.39 | 1.06 |
| Pb3(PO4)2 | ||
| 2.5 mM | 1.01 | – |
| 5.0 mM | 1.00 | 1.01a |
| 10.0 mM | 1.00 | 1.01a |
| Rm:Rc | Rs:Rm | |
| Limescale | ||
| 0.2% (w/v) | 1.19 | 1.67 |
| 0.3% (w/v) | 2.36 | 1.02 |
| 0.4% (w/v) | 2.38 | 1.03 |
| 0.5% (w/v) | 2.39 | 1.06 |
| Pb3(PO4)2 | ||
| 2.5 mM | 1.01 | – |
| 5.0 mM | 1.00 | 1.01a |
| 10.0 mM | 1.00 | 1.01a |
Results are presented in the form of ratios of the rate of growth on amended media in relation to control growth (Rm:Rc) and the rate of extension of the solubilised zone in relation to rate of growth on amended media (Rs:Rm). Control growth rate of A. niger: 7.00 (±0.49) mm day−1 (average of three replicates). aPartial solubilisation only.
3.2 Crystal formation in agar under colonies
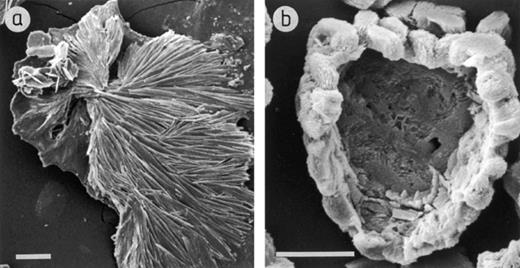
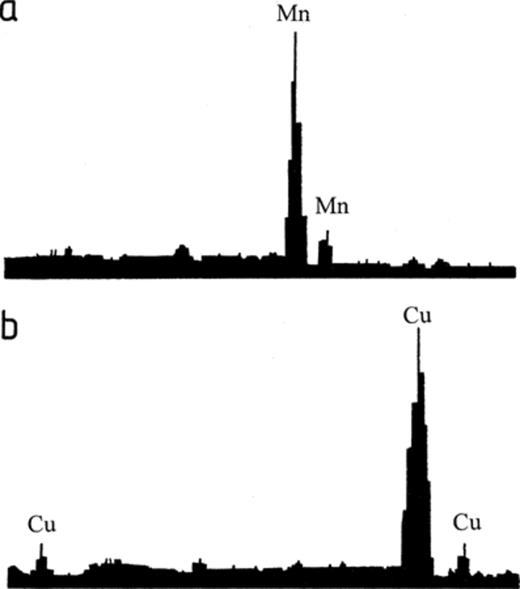
When A. niger was grown on agar amended with cuprite and rhodochrosite, crystals formed in the agar under and around the colonies after 1–2 days growth at 25°C (Fig. 2). The crystals formed under colonies growing on agar amended with rhodochrosite (Figs. 1 and 2a) were fan-like crystal structures (600–800 μm in length) composed of elongated plates of approximately 100–150 μm in length and 30–50 μm in width. The crystals formed under colonies growing on agar amended with cuprite (Fig. 2b) form around the mineral to give a coating of crystal aggregates approximately 7 μm in length and 2 μm in width. X-ray microanalysis of the crystals showed that only the appropriate cation was present in each sample, i.e. manganese for the crystals formed in agar amended with rhodochrosite and copper for the crystals formed in agar amended with cuprite (Fig. 3).
Scanning electron micrographs of crystals purified from the agar under colonies of A. niger growing on MEA amended with (a) 60 mM rhodochrosite, (b) 60 mM cuprite. Scale bars=100 μm and 10 μm respectively.
Typical spectra obtained by energy dispersive X-ray microanalysis of crystals produced by A. niger growing on MEA amended with (a) 60 mM rhodochrosite and (b) 60 mM cuprite.
4 Discussion
In contrast to autotrophic leaching, there has been relatively little attention paid to the heterotrophic solubilisation of insoluble metal compounds (other than metal phosphates) in the natural environment and the incidence of such abilities in microorganisms. There has been some interest, however, in the solubilisation of some naturally occurring metal-bearing minerals. 50% of Ni reserves, for example, are found in lateritic ores and Tzeferis et al. [18] recovered 60% Ni from non-sulfide nickel ores using strains of Aspergillus and Penicillium, probably by the production of citric and oxalic acids and subsequent complex formation. Attempts have also been made to leach Ni from lateritic nickel ore with indigenous microflora (strains of Aspergillus), as microbial leaching was purported to be less energy demanding and less expensive, but it was found that abiotic organic acid applications were more effective than the microbes [19, 20].
The ability of A. niger to solubilise a range of other metal-bearing minerals and limescale has been demonstrated in this work. A. niger solubilised three out of the seven minerals tested, and limescale was solubilised at all concentrations tested. The efflux of protons and the production of organic acids appear to be the most significant for the solubilisation of insoluble metal compounds by A. niger[7, 16]. Growth of A. niger in liquid culture results in major production of organic acids, and typical final concentrations of citric acid produced industrially by A. niger can reach 600 mM [21]. This solubilisation would presumably lead to enhanced mobility and increased availability of Ca2+, Cu2+ and Pb2+, although no reduction in the growth rates of A. niger were recorded. In the terrestrial environment, the dissolution of hematite by simple organic acids (citric and oxalic) occurs by changes in surface micro-topography, such as pitting and pore formation [22]. The presence of acetate (0.07 M) and oxalate (0.005 M) promoted secondary pore development and increased feldspar dissolution rates in sandstones [23], due to the formation of Al-organic acid anion complexes. Organic acids can interact with mineral surfaces in the environment in two ways, either coating the mineral and protecting it from dissolution or increasing rates of dissolution, depending on the nature of the interaction. Hering [24] proposed a mechanism for ligand-promoted dissolution of aluminium oxide: (1) ligand adsorption and surface complex formation, (2) slow detachment of the metal from the surface as a complex with the ligand, (3) regeneration of the surface. Low molecular mass organic acids have been reported in forest litter leachates at a concentration of 1.2 mM, and oxalate concentrations up to 1 mM have been reported in soil solutions [24].
After initial solubilisation of the insoluble metal compound, precipitation of crystals in the agar under the colonies growing on cuprite and rhodochrosite has been demonstrated. Crystals of this nature have been observed under colonies growing on agar amended with a range of insoluble metal phosphates and oxides and have previously been identified as insoluble metal oxalates [7]. The crystals reported here are therefore likely to be manganese and copper oxalates and EDXA showed them to contain only the relevant metal. While calcium oxalate is known to be degraded only by anaerobic bacteria of the gastrointestinal tract and by a few aerobic actinomycetes, bacteria and fungi [25], there is little information on the degradation of insoluble oxalates containing potentially toxic metal cations. The formation of metal oxalates could therefore be an effective means of immobilisation of toxic metal ions, and has implications for tolerance [7].
In this study, A. niger appeared not to be able to solubilise lead phosphate, and it is claimed that if lead is present in soil as lead phosphate it has a limited bioavailability [14]. Growth of the fungus was not affected by the presence of lead phosphate in the medium. A. niger will produce organic acids and acidify the medium whether a metal compound is present or not [7], and it is possible that some complexation could have occurred. In addition, it should be noted that any solubilised Pb2+ would have readily precipitated with other medium components, e.g. chloride [2]. However, PbO has been solubilised with Clostridium sp. by the production of acetic, butyric and lactic acids [15].
In conclusion, this work has demonstrated the ability of A. niger to tolerate and solubilise a range of metal-bearing minerals when incorporated into MEA. The production of organic acids provides both protons and an organic acid anion, the latter capable of forming a complex with the metal cation, and, in some cases the subsequent formation of an insoluble oxalate. The environmental significance of this latter process of immobilisation awaits further study.
Acknowledgements
G.M.G. gratefully acknowledges financial support from the Biotechnology and Biological Sciences Research Council (SPC 02812) and NATO (Linkage Grant Envir. Lg. 950387). J.A.S. gratefully acknowledges receipt of a NERC postgraduate research studentship. Dr. F. Hubbard, Department of Civil Engineering, University of Dundee is thanked for assistance with powder X-ray diffraction of the minerals.
References
Morley, G.F., Sayer, J.A., Wilkinson, S.C., Gharieb, M.M. and Gadd, G.M. (1996) Fungal sequestration, mobilization and transformation of metals and metalloids. In: Fungi and Environmental Change (Frankland, J.C., Magan, N. and Gadd, G.M., Eds.), pp. 235–256. Cambridge University Press, Cambridge.
Hering, J.G. (1995) Interaction of organic matter with mineral surfaces. Effects of geochemical processes at the mineral-water interface. In: Aquatic Chemistry – Interfacial and Interspecies Processes (Huang, C.P., O'Melia, C.R. and Morgan, J.J., Eds.), pp. 95–110. American Chemical Society, Washington, DC.