-
PDF
- Split View
-
Views
-
Cite
Cite
Chika Hashimoto, Masayuki Hashimoto, Hirofumi Honda, Jun-ichi Kato, Effects on IS1 transposition frequency of a mutation in the ygjD gene involved in an essential tRNA modification in Escherichia coli, FEMS Microbiology Letters, Volume 347, Issue 2, October 2013, Pages 140–148, https://doi.org/10.1111/1574-6968.12227
Close - Share Icon Share
Abstract
The YgjD protein is essential for the synthesis of the universal tRNA modification, N6-threonylcarbamoyladenosine (t6A), which is necessary for the decoding of ANN codons. We isolated a suppressor (ygjDsup) of the ygjDts mutant by its permissive growth at high temperature in Escherichia coli. Resequencing of the ygjDsup mutant genome showed the presence of a complicated chromosome rearrangement, an inverse insertion of a large duplicated region (c. 450 kb) into a small deleted region. The temperature-resistant growth associated with ygjDsup was due to the presence of multicopy suppressor genes, yjeE and groL, of the ygjDts mutation in the duplicated region. This DNA rearrangement was not simply mediated by IS1 transposition, but the duplicated region was flanked by IS1. We showed that the frequency of IS1 transposition was increased in ygjDts mutants. The transposase of IS1 is coded for by the insB gene, and its translation occurs through a frameshift of a ribosome translating upstream of the insA gene. We showed that this frameshifting frequency was increased by the ygjDts mutation. These results indicated that the mutation of the gene for tRNA modification, t6A, affected IS1 transposition.
Introduction
The universal YgjD/Kae1/Qri7 protein family is conserved among eukaryotes, Archaea, and bacteria (Galperin & Koonin, 2004), and it is involved in the synthesis of the tRNA modification, N6-threonylcarbamoyladenosine (t6A; El Yacoubi et al., 2011; Srinivasan et al., 2011; Deutsch et al., 2012; Lauhon, 2012; Perrochia et al., 2013). The t6A modification is present at position 37 of all tRNAs that pair with ANN codons and is necessary for decoding ANN codons (Murphy et al., 2004). The biological processes, which the factors involved in this t6A modification participated in, and their functions in those processes have not been clarified. The ygjD yeast ortholog, kinase-associated endopeptidase 1 (kae1), encodes a component of a complex known as kinase, endopeptidase, and other proteins of small size (KEOPS)/endopeptidase-like kinase chromatin-associated (EKC). Several phenotypes have been associated with mutants of this complex: short telomeres, defective transcription of several genes encoding proteins involved in cell cycle progression and polarized growth, and genomic instability (Downey et al., 2006; Kisseleva-Romanova et al., 2006; Oberto et al., 2009; Daugeron et al., 2011). In KEOPS/EKC mutants, defective t6A tRNA modification affected translation initiation at the open reading frames (ORFs) in the upstream region of GCN4, which encodes a transcriptional activator (Daugeron et al., 2011). Because these ORFs function in inhibition of GCN4 translation, GCN4 derepression was provoked, and the transcription of GCN4p targets increased. It is possible that the pleiotropic phenotypes associated with KEOPS/EKC mutants are the indirect result of the defective t6A modification.
In prokaryote and mitochondria, at first, the ygjD ortholog in Mannheimia (formerly Pasteurella) haemolytica was found to encode an O-sialoglycoprotein endopeptidase (Gcp; Abdullah et al., 1991). The studies of YgjD function in other organisms have suggested roles in DNA maintenance. In the Archaea Pyrococcus abyssi, the ygjD ortholog was reported to possess apurinic endonuclease activity (Hecker et al., 2007). But this activity could not be reproduced (Mao et al., 2008). The ygjD ortholog Qri7 has been implicated in mitochondrial genome maintenance in both Saccharomyces cerevisiae and Caenorhabditis elegans. It remains to be determined whether Qri7 functions directly in genome maintenance or indirectly through altered translation caused by a deficiency of the tRNA modification (Oberto et al., 2009). The Qri7 has been required for embryo development in Arabidopsis thaliana (Haussuehl et al., 2009).
In Escherichia coli, essential YgjD functionally interacts with YeaZ and YjeE, both of which are also essential for cell growth and are conserved in Eubacteria (Arigoni et al., 1998; Freiberg et al., 2001; Teplyakov et al., 2002; Allali-Hassani et al., 2004; Butland et al., 2005; Nichols et al., 2006; Kato & Hashimoto, 2007; Lerner et al., 2007; McCutcheon & Moran, 2007; Handford et al., 2009; Srinivasan et al., 2011). The formation of ternary complex YgjD–YeaZ–YjeE was calorimetrically proved (Nichols et al., 2013). Their functional interaction was first suggested because strains that conditionally express these three genes were complemented by a multicopy plasmid carrying the rstA gene, which codes for a response regulator of a two-component system (Handford et al., 2009). The functional interaction was also suggested by the results of multicopy suppression: the yeaZts mutation was suppressed by yjeE or ygjD multicopy plasmids, the ygjDts mutant was suppressed by yjeE or yeaZ multicopy plasmids, and the yjeEts mutant was suppressed by the yeaZ multicopy plasmid (Hashimoto et al., 2011). Physical interactions between YeaZ and YjeE and between YeaZ and YgjD and the proteolysis of purified YgjD mediated by purified YeaZ were shown (Handford et al., 2009). But this proteolysis could not be reproduced by other group (Nichols et al., 2013). Furthermore, the four proteins (YgjD, YrdC, YjeE and YeaZ) were shown to be both necessary and sufficient for t6A biosynthesis in vitro (Deutsch et al., 2012). In ygjDts mutants, transcription of the thr and ilv operons was increased indirectly through the inhibition of transcriptional attenuation by defective translation of the leader peptides (Hashimoto et al., 2011).
In this work, to identify and characterize the phenotypes associated with the ygjDts mutant, a suppressor mutant (ygjDsup) was isolated from the ygjDts mutant. Molecular genetic analyses of ygjDsup revealed that the IS1 transposition frequency was increased by the ygjDts mutation.
Materials and methods
Strains, plasmids and culture media
All E. coli strains used are derivatives of MG1655 except for ME8434 [NBRP (NIG, Japan): E. coli] and DH1 (Hanahan, 1983). Isolation of the ygjDts mutant was described previously (Hashimoto et al., 2011). Plasmids used were pSG76A, pSG76K, pPIR-K (Pó sfai et al., 1997), pSG76A2 (Iwadate et al., 2011) and pACYC184 (Chang & Cohen, 1978). The E. coli chromosome library used was that constructed using pACYC184 as a vector (Kato & Katayama, 2001). Bacteria were grown routinely in LB broth and Antibiotic Medium 3 (Becton Dickinson) broth or on Antibiotic Medium 3 plates. Where relevant, antibiotics were added to concentrations of 50 μg mL−1 ampicillin (Ap), 50 μg mL−1 kanamycin (Km), and 15 μg mL−1 chloramphenicol (Cm).
Isolation of the ygjDsup mutant
The ygjDts mutant [MG1655 ΔygjD:Sm ΔrecA:Tc/mini-F-ygjDts1(Ap)] was grown at 42 °C on Antibiotic Medium 3 plates. The temperature-resistant colonies obtained spontaneously were checked for growth at 25 °C on Antibiotic Medium 3 plates, and we could isolate only one cold-sensitive strain (sup 1–5–1).
Resequencing of the ygjDsup mutant genome
Cells were grown overnight at 37 °C in Antibiotic Medium 3. The ygjDsup mutant genome was isolated using DNeasy (Qiagen) and sequenced with the Genome Analyzer GAIIx (Illumina). The sequencing data were 31 117 579 reads (38 bases per read).
Cloning and sequencing the junction regions of the insertion/deletion in the ygjDsup mutant genome
The chromosome regions, A and B, flanking a deletion identified by resequencing, were amplified by PCR using oligonucleotides 500-35 and 500-36, and 500-32 and 500-34 (Table S1, Supporting information) as primers, respectively, and cells of the ygjDsup mutant as a template. The DNA fragments, A and B, were digested with PstI and SacI, and SphI and EcoRI and ligated with the PstI and SacI, and SphI and EcoRI digests of pSG76A2, respectively. The pSG76A2 (Ap) vector cannot replicate without pPIR-K, which is a temperature-sensitive plasmid with the pir gene necessary for replication of pSG76A2. Each of the resultant plasmids and pPIR-K were introduced into the ygjDsup mutant, which was transformed by pACYC184-ygjD+ and mini-F-recA+ (Km), resulting in the loss of the mini-F-ygjDts1(Ap) plasmid of the original ygjDsup mutant (Fig. S1). The ApR strains, in which the pSG76A2 derivative plasmids were integrated into the chromosome of the ygjDsup mutant by homologous recombination at A or B region, were obtained at 42 °C and checked by PCR. These ApR strains for the A and B regions were grown overnight in Antibiotic Medium 3, and their genomic DNA was prepared by DNeasy (Qiagen). The purified DNA samples of regions A and B were digested with SacI and SphI, respectively, ligated, and introduced into the E. coli strain, DH1, together with pPIR-K at 30 °C. The resultant plasmids were sequenced with the oligonucleotides, 500-30, 500-29, and 495-1 (Table S1), as primers, and both junction sequences of the chromosomal deletion were determined.
Construction of gene disruptants
Linear DNA fragments containing the CmR gene flanked with the 40-bp regions flanking the coding regions of yjhU, yjhF, yjhG, tatD, fadA, ppc, malK, mgtA, idnT, groS-groL, and lacI-lacZ were generated by PCR with oligonucleotides, 500-9 and 500-10, 500-11 and 500-12, 500-13 and 500-14, tatD-CmN and tatD-CmC, fadA-CmN and fadA-CmC, ppc-CmN and ppc-CmC, malK-CmN and malK-CmC, mgtA-CmN and mgtA-CmC, idnT-CmN and idnT-CmC, groES-CmN and groEL-CmC, lacI-CmN and lacA-CmC, as primers (Table S1), respectively, and pACYC184 as a template. These fragments were introduced into the E. coli strain MG1655 red by electroporation, and CmR recombinants were isolated. The construction of the deletion mutation of the chromosomal yjeE gene in the presence of the complementing plasmid was previously described (Hashimoto et al., 2011). The groS-groL deletion was constructed in this work using the complementing plasmid, pR16-3 (Kato & Hashimoto, 2007; for details, see a deletion, OCR16-4, in the database, PEC http://www.shigen.nig.ac.jp/ecoli/pec/index.jsp). The chromosome regions containing the resultant disrupted genes were introduced into the ygjDsup mutant and the control strain, MG1655, by P1 transduction. For disruption of the genes of the ygjDsup mutant, the ygjDsup mutant transformed by the plasmids, pACYC184-ygjD+ and mini-F-recA+ (Km), was used as a recipient of P1 transduction.
Pulse-field gel electrophoresis
Pulse-field gel electrophoresis (PFGE) was performed using the CHEF-DR II system (Bio-Rad) with 1% PFC agarose (Bio-Rad) in 0.5× TBE buffer. The running time was 22 h; voltage, 200 V; and ramping, 10–30 s.
Assay of Tn9 (IS1) transposition frequency
The E. coli strain, ME8434, which includes Tn9 in the genome (zbj-274::Tn9), was grown overnight at 37 °C in Antibiotic Medium 3. Genomic DNA was prepared using DNeasy (Qiagen), digested with BamHI and BglII, ligated with the BamHI digest of pSG76K, and introduced into the strain, DH1, together with pPIR-K (Km) at 30 °C. The Cm-resistant colony was selected, and the pSG76K-Tn9 was obtained from this colony. This pSG76K-Tn9 (Cm) plasmid was introduced into the ygjDts and the ygjD+ strains together with pPIR-K. The transformants were grown at 30 °C for 24 h after inoculation into Antibiotic Medium 3 broth. The cultures were 10−6 diluted into fresh Antibiotic Medium 3, of which 100 μL was incubated on Antibiotic Medium 3 plates with or without Cm at 40 °C for 4 days. At 40 °C, the ygjDts mutant grows slowly, and the pPIR-K plasmid hardly replicates. Colonies were incubated on Antibiotic Medium 3 plates containing both Cm and Km or Cm only and were confirmed to be CmR KmS. The ratio of the number of CmR KmS colonies to that of the colonies on plates without Cm at 40 °C was regarded as the transposition frequency.
Assay of insA–insB frameshifting in IS1 using IS1–lacZ fusion plasmids
The IS1–lacZ fusion plasmids, pSEK9000 Km and pSEK9000-I Km, were constructed by inserting a KmR fragment into the ScaI site within the ApR marker of pSEK9000 and pSEK9000-I, respectively (Sekine et al., 1992). Each of those plasmids was introduced into the ygjDts mutant [MG1655 ygjD:SmR ΔrecA:Tn10 Δ(lacI-lacA):CmR/mini-F (ApR)-ygjDts] and the isogenic ygjD+ strain [MG1655 ygjD:SmR ΔrecA:Tn10 Δ(lacI-lacA):CmR/mini-F(ApR)-ygjD+]. The stationary phase culture of those transformants grown at 30 °C in LB containing Km was 10−2 diluted into fresh LB containing Km and incubated at 30 °C for 1 h and then at 42 °C for 2 h. The β-galactosidase activity was measured as described in the study by Miller (1972).
Cloning of dinG and rep
The dinG and rep DNA fragments were amplified by PCR with oligonucleotides 182-34 and 182-35, and 454-59 and 454-60 as primers (Table S1), respectively. The fragments were then digested with BamHI and inserted into the BamHI site of pACYC184 to obtain the dinG and the rep multicopy plasmids.
Results and discussion
Isolation and characterization of a suppressor of the ygjD temperature-sensitive mutant
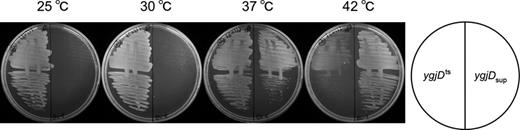
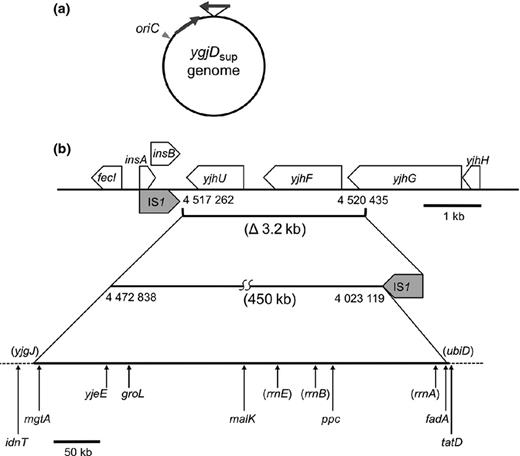
We isolated a suppressor mutant (ygjDsup) from the ygjDts mutant, the latter of which was isolated in our previous work (Hashimoto et al., 2011). Among the spontaneously obtained temperature-resistant strains, a cold-sensitive mutant was selected to identify easily a suppressor gene (Fig. 1). We introduced an E. coli chromosome library into the ygjDsup mutant and isolated plasmids, which corrected the cold-sensitive growth of this mutant. We isolated the dinG plasmids, but these plasmids did not render the ygjDsup mutant sensitive to high temperatures. Consistent with this, DNA sequence analysis showed that the dinG gene was not mutated in the ygjDsup mutant, indicating that this was not the suppressor gene. Then, we resequenced the genome of the ygjDsup mutant. The results of this resequencing and the confirmative sequencing analyses indicated that there was not a point mutation, but only one insertion/deletion mutation in the 4 517 262–4 520 435 region (Fig. 2).
Growth of the ygjDts and the ygjDsup mutants. The mutants were incubated on Antibiotic Medium 3 at 25, 30, 37, or 42 °C.
DNA rearrangement in the ygjDsup mutant. (a) The arrows represent the duplicated chromosome region. (b) The duplicated region inserted in an inverse orientation into the chromosome region is indicated by the straight lines. The c. 3.2-kb yjhU–yjhG chromosomal region (4 517 262–4 520 435) was deleted, and the c. 450-kb mgtA–fadA chromosomal region (4 023 119–4 472 838) was inversely inserted into the deleted region. The duplicated region was flanked by two copies of IS1 (gray arrows).
To characterize this insertion/deletion mutation, some additional experiments were needed. First, PCR was carried out using primers flanking the potential deletion; this did not result in the identification of a deletion in the ygjDsup mutant genome. Introduction of a newly constructed yjhU–yjhG deletion mutation into the ygjDts mutant did not affect the latter's temperature-sensitive growth, indicating that this deletion mutation was not the suppressor mutation. To identify the junction sequences of the suppressor deletion, flanking chromosomal regions on the 5′ and 3′ sides of the deletion were amplified by PCR and cloned into a R6K derivative plasmid, pSG76A2, which does not replicate due to the lack of a pir gene. Each of the resultant plasmids was introduced into the ygjDsup mutant and the strains and incubated at 42 °C, in which the pSG76A2 derivative plasmids were stably introduced into the chromosome of the ygjDsup mutant by homologous recombination (Fig. S1). Their chromosomal DNA was prepared, digested with restriction enzymes, ligated, and introduced into the E. coli strain together with a helper plasmid with the pir gene, pPIR-K, at low temperature to obtain the plasmids carrying the junction regions. Their DNA sequence analysis revealed that a c. 450-kb region (4 023 119–4 472 838) was inversely inserted into the deleted region (Fig. 2).
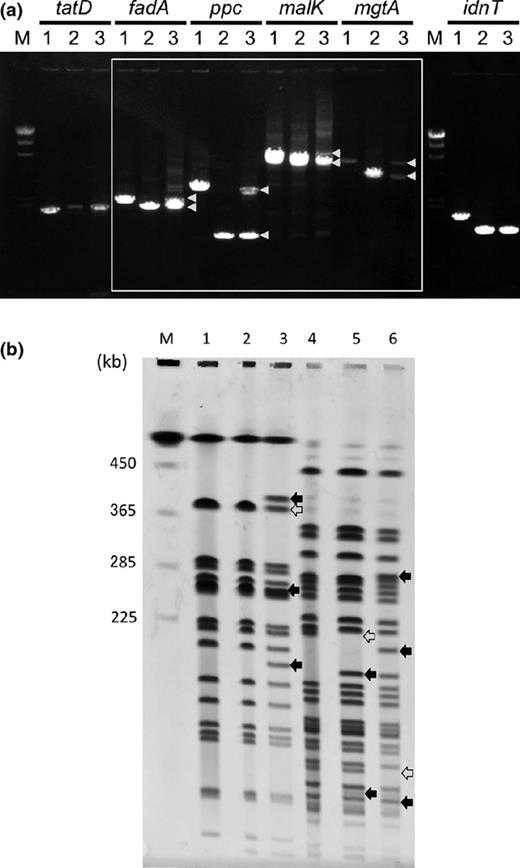
An approximate twofold increase in sequencing redundancy for this c. 450-kb region suggested that this region was duplicated. To confirm the duplication, we first constructed disruptants of each of the fadA, ppc, malK, and mgtA genes from the duplicated region, together with the tatD and idnT genes, which lie in the flanking regions of the deletion, using the MG1655 red strain. Each disrupted gene was introduced into the ygjDsup mutant by P1 transduction. PCR analyses of the transductants showed that two bands, one for the disrupted genes and the other for the intact (undisrupted) genes, were detected within the duplicated region, but only one band for the disrupted gene was detected within the flanking regions (Fig. 3a).
Confirmation of the DNA rearrangement in the ygjDsup mutant. (a) PCR analysis of the duplicated region. The fadA, ppc, malK, and mgtA genes are included in the duplicated region, but the tatD and idnT genes are not. The white frame indicates the band pattern of the genes within the duplicated region. A pair of DNA fragments represented by arrowheads, one for the intact genes and the other for the disrupted genes, was amplified by PCR for each of the genes in the duplicated region, although only one band for each of the genes outside the duplicated region was detected. The cells used as PCR templates were as follows: MG1655 (lane 1); MG1655 with disrupted gene (lane 2); the ygjDsup mutant with disrupted gene (lane 3). (b) PFGE analysis of the ygjDts and ygjDsup mutants. Chromosomal DNA was digested to completion with NotI or XbaI and analyzed by PFGE. The DNA was visualized by ethidium bromide staining and UV irradiation. Lane 1, MG1655, NotI; lane 2, the ygjDts mutant, NotI; lane 3, the ygjDsup mutant, NotI; lane 4, MG1655, XbaI; lane 5, the ygjDts mutant, XbaI; lane 6, the ygjDsup mutant, XbaI; M, DNA size marker (Saccharomyces cerevisiae chromosomal DNA). Closed and open arrows indicate the extra and missing bands, respectively. The patterns match those predicted from the genome sequences except for ΔrecA::Tn10 of the ygjDts and the ygjDsup mutants (Fig. S2). Because Tn10 has an XbaI site, a 206 624-bp fragment was split to c. 171-KB and c. 45-kb bands. In the presence of Dam methylase, two XbaI sites (3 861 916 and 4 308 586) are not digested by XbaI.
Next, the DNA rearrangement of the ygjDsup mutant was confirmed by PFGE. The NotI and XbaI digests of chromosomes of the ygjDsup mutant and its parental strain were analyzed (Fig. 3b). Consistent with the predictions, three new bands appeared and one band disappeared in NotI digests of the ygjDsup mutant chromosome (Fig. 3b and Fig. S2). As for the XbaI digests, two new bands appeared and one band disappeared in the ygjDts mutant chromosome, which can be attributed to the ΔrecA::Tn10 mutation. Consistent with the predictions for the Dam-methylated chromosome, three new bands appeared and one band disappeared in the XbaI digest of the ygjDsup mutant chromosome (Fig. 3b and Fig. S2). These results directly confirmed the DNA rearrangement associated with the ygjDsup mutation.
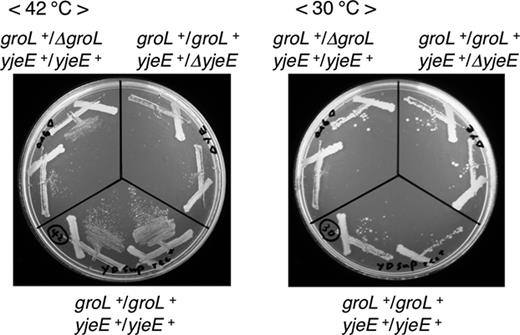
The isolated ygjDsup suppressor mutant grew at high temperature. How is the temperature-sensitive growth of the ygjDts mutation suppressed by the DNA rearrangement in ygjDsup? In the duplicated region, there exists a multicopy suppressor gene, yjeE, described previously (Hashimoto et al., 2011). In addition to yjeE, the groS-groL genes in the duplicated region were identified as multicopy suppressors in a survey of temperature-resistant cells by introducing an E. coli chromosome library into the ygjDts mutant (Y. Taniguchi & J. Kato, unpublished). To examine the effects of their duplication on the growth of the ygjDsup suppressor mutant, the disrupted yjeE and groS-groL genes were introduced into the mutant. These genes are essential for cell growth; therefore, those can be disrupted in the presence of the complementing plasmids for the wild-type strain. When this ygjDsup suppressor mutant was used as recipient of P1 transduction, a number of transductants with their deletions appeared due to duplication of these genes. The growth of the Δ(groS-groL) and ΔyjeE derivatives constructed at 37 °C was examined at 42 and 30 °C (Fig. 4). At 42 °C, both deletion derivatives grew slower than the ygjDsup suppressor mutant, indicating that duplication of those genes was responsible for temperature-resistant growth. Although these multicopy suppressor genes were only duplicated, the duplicated genes were inserted into the chromosome region not far from oriC, and if their copy numbers increase more than twofold during cell proliferation, then both genes together may account for multicopy suppression.
Growth of the yjeE or groS-groL deletion derivatives of the ygjDsup mutants. To introduce the deletion mutations into the ygjDsup suppressor mutant by P1 transduction, the recA− mutation of the ygjDsup mutant had to be repaired. Because the ygjDsup suppressor mutant was isolated from the ygjDts mutant constructed by plasmid shuffling, the chromosomal ygjD gene was disrupted in the presence of the mini-F plasmid (Ap) with the ygjDts mutant gene. Then, the recA+ gene was cloned into the mini-F plasmid (Km) with the ygjDts mutant gene, and the complementing mini-F plasmid (Ap) with the ygjDts mutant gene was replaced with the resultant mini-F plasmid (Km) with the ygjDts mutant gene and the recA+ gene. The ygjDsup mutant (groL+/groL+yjeE+/yjeE+) and its deletion derivatives (groL+/ΔgroL yjeE+/yjeE+, groL+/groL+yjeE+/ΔyjeE) were incubated on Antibiotic Medium 3 at 30 or 42 °C.
The ygjDsup mutant showed not only temperature-sensitive growth, but also cold-sensitive growth. Both of the yjeE and the groS-groL deletion derivatives did not grow well at 30 °C; therefore, the cold-sensitive growth of the ygjDsup suppressor mutant was not ascribed to the duplication of the yjeE, groS, and groL genes (Fig. 4). How does the DNA rearrangement cause the cold-sensitive growth? As described above, we isolated the dinG plasmids, which corrected the cold-sensitive growth, from the E. coli chromosome library. Because the viability of the strain, in which rrn operons are inverted to face replication, is affected by the overproduction of helicases or RNase H (Boubakri et al., 2010), we examined the effects of the multicopy plasmids carrying one of other helicase genes, rep and uvrD, and rnhA on the growth of the ygjDsup mutant. The dinG plasmid strongly, and the rep and the rnh plasmids weakly, suppressed cold-sensitive growth of the ygjDsup mutant although the growth was not affected by the uvrD plasmid (Fig. S3). The duplicated and inversely inserted chromosome region in the ygjDsup mutant contains three rrn operons. The rrn inversion is presumed to be unstable due to the head-on collision between DNA replication and transcription complexes (Schmid & Roth, 1983; Brewer, 1988; Boubakri et al., 2010). The results of this work suggested that the cold-sensitive growth of the ygjDsup mutant may be due to collision between replication and transcription complexes. The fact that the ygjDsup mutant grew at high temperature suggests that the ygjDsup mutation may make the strain tolerant to the collision; that is, that cell growth was inhibited by that collision in the presence of YgjD activity. Our results lay the foundation for investigating whether YgjD may be indirectly involved in genome integrity through tolerance to the collision. We have previously examined the transcriptional profiles of the ygjDts mutant at 30 and 37 °C (Hashimoto et al., 2011). The transcriptional level was not clearly increased at 37 °C for rnhA, dinG and mfd, which encodes a transcription–repair coupling factor promoting direct restart of the fork after the collision by facilitating displacement of RNAP (Table S2; Pomerantz & O' Donnell, 2010). It is difficult to explain by increase in transcription of the relevant factors. Further investigation will be necessary to clarify the cold sensitivity of the ygjDsup mutant.
Increased IS1 transposition frequency in the ygjD temperature-sensitive mutant
The DNA rearrangement identified in the ygjDsup mutant consists of an inverse insertion of a duplicated region into a short deleted region. How did this DNA rearrangement occur in the ygjDts mutant? The duplicated region was flanked by two copies of IS1. One of them is an extra copy, and they formed an inverted repeat. The inversion of the duplicated region may be due to homologous recombination between these two copies of the inverted repeat. If two copies of IS1 were transposed into the sites, 4 472 838 and 4 023 119, and the regions flanked by the IS1 sequences were duplicated in association with excision and transposition of the huge transposon-like structures into the yjhG gene, the yjhU–yjhF–yjhG short deletion could be explained by homologous recombination between the two copies of the IS1 direct repeat that originally existed between fecI and yjhU, and the new IS1 inserted within yjhG (Fig. 2). This would also explain the absence of a 9-bp target duplication of IS1 at the junctions, insB–yjgJ, yjhG–insA, and ubiD–insB (Fig. S4).
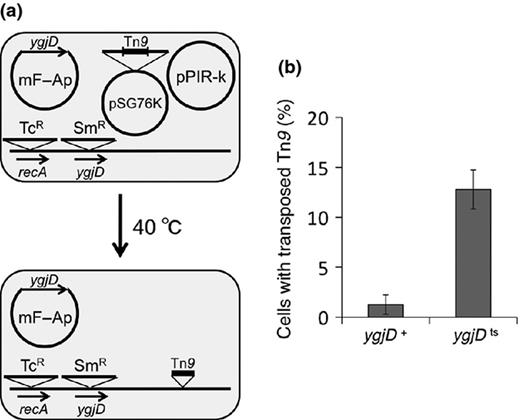
Although the details of the recombination events have not been clarified, the long duplicated and inversely inserted region was flanked by IS1, led to the hypothesis that IS1 transposition frequency may increase due to the ygjDts mutation (Fig. 2). To examine the IS1 transposition frequency, we cloned Tn9, which consists of the chloramphenicol resistance gene flanked by two copies of IS1, into a R6K derivative plasmid, pSG76K. The resultant plasmid, pSG76K-Tn9, was introduced into the ygjDts mutant and the isogenic ygjD+ strain, together with the pPIR-K, and the transformants were incubated at 40 °C, which is the semi-permissive temperature for the ygjDts mutation (Fig. 5a). After incubation for 4 days, the transposition frequency was determined from the number of CmR colonies, as described in Materials and methods. The results showed that the Tn9 (IS1) transposition frequency was about 10.1-fold higher in the ygjDts mutant than in the control ygjD+ strain (Fig. 5b).
Tn9 (IS1) transposition frequency in the ygjDts mutant. (a) A plasmid carrying Tn9, pSG76K-Tn9, and a helper plasmid, pPIR-K, which has the pir gene required for replication of pSG76K-Tn9 and cannot replicate at 40 °C, were introduced into the ygjD+ and the ygjDts strains. Transformants were incubated on Antibiotic Medium 3 plates with or without Cm at 40 °C for 4 days. 40 °C is the semi-permissive temperature for the ygjDts mutant. The pSG76K-Tn9 plasmid first replicated owing to pPIR-K and then stopped replicating due to the absence of pPIR-K. Therefore, the CmR strains obtained at 40 °C had Tn9 transposed into the genome. (b) The transposition frequency is the ratio of the number of CmR KmS colonies on plates containing Cm to that of the colonies on plates without Cm at 40 °C. The Tn9 transposition frequencies (%) of the ygjD+ and the ygjDts mutants were 1.26 ± 0.97 and 12.78 ± 1.98, respectively. The data represent the average of three independent experiments.
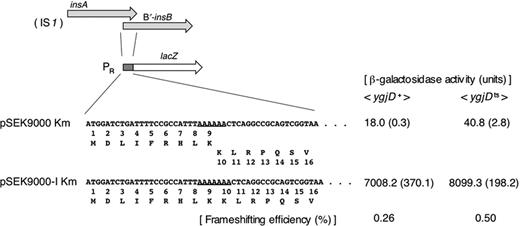
To elucidate the mechanism by which the IS1 transposition frequency was increased in the ygjDts mutant, the expression of the IS1 transposase was examined. The transposase of IS1 is coded for by the insB gene, and its translation occurs through a frameshift of a ribosome translating upstream of the insA gene (Sekine & Ohtsubo, 1989; Sekine et al., 1992). Therefore, we examined the effects of the ygjDts mutation on this frameshifting with the pSEK9000 plasmid, which has the insA–B′-insB–lacZ fusion gene under the lambda PR promoter. The KmR derivatives of this plasmid and the control plasmid with the control in-frame fusion gene were introduced into the ygjDts mutant, and the β-galactosidase was assayed after 2-h incubation at 42 °C. The expression of the IS1–LacZ fusion protein was more than twice higher for the ygjDts mutant than for the control ygjD+ strain (Fig. 6). These results indicated that the frameshift occurs more frequently in the ygjDts mutant. The frameshifting occurs within a run of adenines in the insA–B′-insB region and may be affected by the deficiency of ANN codon decoding activity.
Structures of IS1–lacZ fusion plasmids and the activity of β-galactosidase produced in cells harboring each of the plasmids. Two arrows at the top indicate the overlapping ORFs, insA and B′-insB, of IS1. The pSEK9000 Km and pSEK9000-I Km plasmids have IS1–lacZ fusion genes, and they are transcribed by bacteriophage lambda PR promoter, which is controlled by a temperature-sensitive repressor, the product of cI857 carried by those plasmids. The DNA fragment of wild-type IS1 containing the run of adenines (underlined) is flanked by the initiation codon of bacteriophage lambda cro gene and the lacZ gene on the pSEK9000 Km plasmid. The occurrence of −1 frameshifting during translation of the run of adenines results in the synthesis of the InsA–B′-InsB–LacZ fusion protein. The IS1 DNA fragment of the control plasmid, pSEK9000-I Km, has a single adenine insertion in the run of adenine (underlined), so that insA is fused with B′-insB–lacZ in-frame. The ygjDts mutant and the isogenic ygjD+ strain were transformed with each of pSEK9000 Km and pSEK9000-I Km. Those transformants were grown, and their β-galactosidase activities were assayed as described in Materials and methods. Because the strains were disruptants of lac operon, the background noise level for the β-galactosidase activity of the ygjDts mutant and the isogenic ygjD+ strain was negligible.
In this study, we showed that YgjD is indirectly involved in controlling transposition frequency. Further analyses are necessary to investigate the other phenotypes of the ygjD mutation. To understand the various functions of YgjD, it is important to clarify whether or not these phenotypes are the direct results of defective t6A modification.
Acknowledgements
We thank Dr Yasuhiko Sekine for kindly sending us plasmids, pSEK9000 and pSEK9000-I, NBRP (NIG, Japan): E. coli for a strain, ME8434, and Dr Nobuhisa Furuya for helpful discussion. This work was supported by Grant-in-Aid for Scientific Research (KAKENHI) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Cloning of the junction regions of the isertion/deletion in the ygjDsup mutant genome.
Fig. S2. The PFGE patterns predicted from the genome sequences.
Fig. S3. Growth of the ygjDsup mutant with the dinG, rep, and rnhA multi-copy plasmids.
Fig. S4. Junction sequences of the insertion/deletion in the ygjDsup mutant genome.
Table S2. Transcription of collision-related genes in the ygjDts mutant.
Author notes
Editor: Wolfgang Schumann